Our Story
Mark Moore, a Certified Registered Nurse Anesthetist (CRNA) and Founder/CEO of Prep Tech, has been working in the medical industry for over 36 years. Moore has made it part of his life’s mission to develop innovative methods for improving the way he and fellow healthcare professionals can work smarter and safer in operating rooms.
To bring innovative medical devices to market, Moore formed a team of healthcare professionals including Co-founder & Chief Medical Officer Erich Wolf, MD, PhD, Biomedical Engineer and Neurosurgeon. After securing several Utility Patents for ULTRAPREP™, Moore and Wolf expanded their team to include select medical advisors, physicians, manufacturers, legal consultants, and other business professionals.
ADVENT OF ISOCUBE™
Since the Taiwan-based plexiglass “aerosol box” impetus in March 2020, PrepTech has created a series of protective barrier devices, now patent-pending and being manufactured around the US.
In May 2020, the FDA released a “generic” Emergency Use Authorization (EUA) of “Protective Barrier Enclosures” designed with specific guidelines to protect healthcare workers. Trademarked as ISOCUBE™, Prep Tech’s barriers conformed to the FDA’s EUA guidelines and were finally authorized for sale in the US through an FDA EUA.
Two main ISOCUBE™ devices being commercialized by Prep Tech, including a multi-use stainless-steel (ISOCUBE™ SS) frame/rail system with disposable isolettes; and a fully disposable, single use, wire framed device (ISOCUBE™ ONE) with similar disposable isolettes as the SS units. Single-use devices are perfect for healthcare facilities, medical transportation, industrial plants, and other facilities that do not wish to disinfect the ISOCUBE™ frames after each use.
ULTRAPREP™ CLINICAL TRIALS AND COVID-19
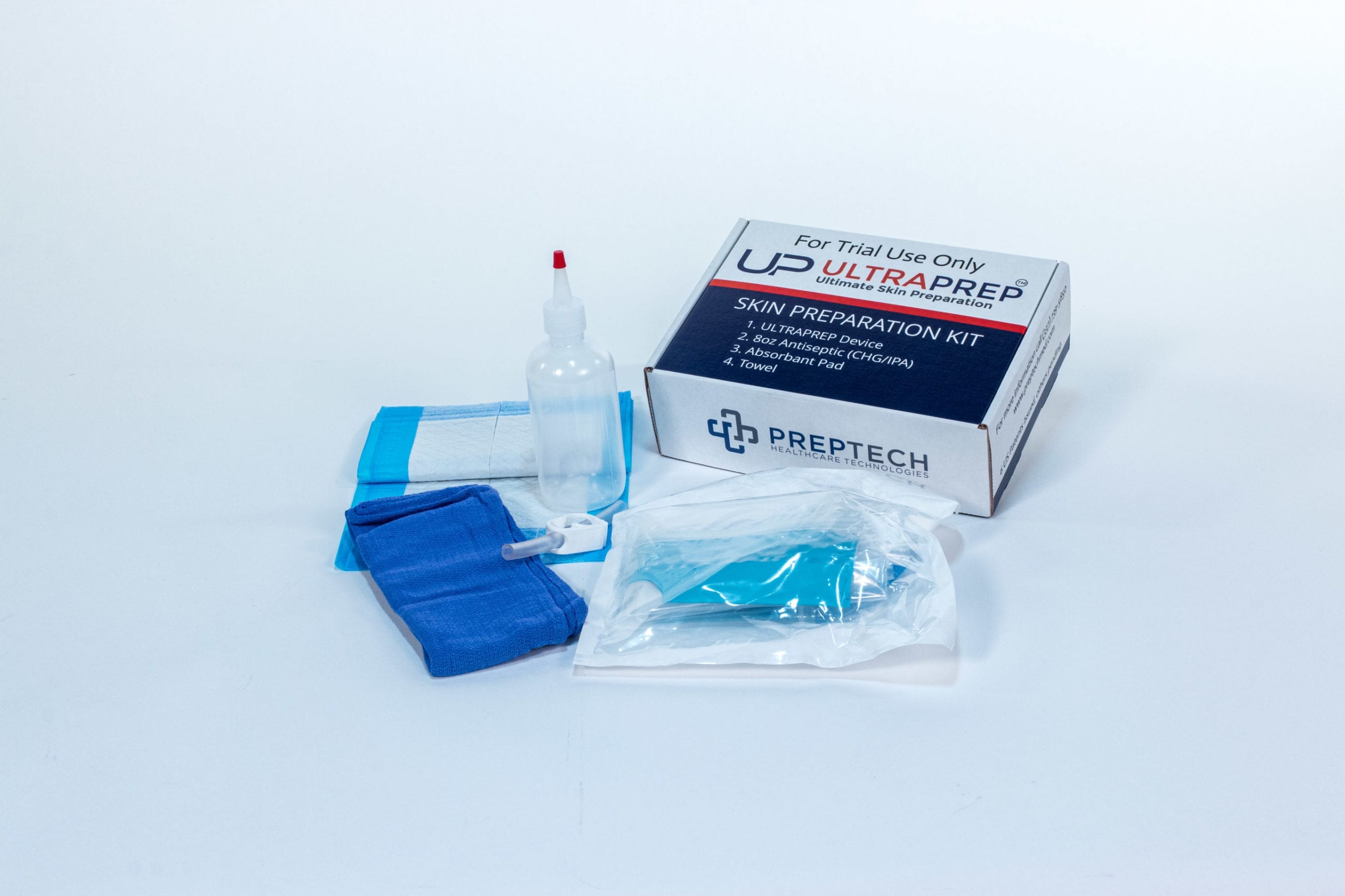
The company’s first device, ULTRAPREP™, is registered with the FDA as a Class 1 exempt medical device and are now available for purchase from Prep Tech and through select distributors.
In a 114-patient clinical trial on extremity surgeries, ULTRAPREP™ proved to save 6-9 minutes per surgery by shifting OR skin prep to outside the OR. For hospitals focused on better healthcare outcomes, this can mean over 15% – 30% increase in OR efficiencies for more surgeries to be done per day/week or allowing medical teams to complete work days faster.
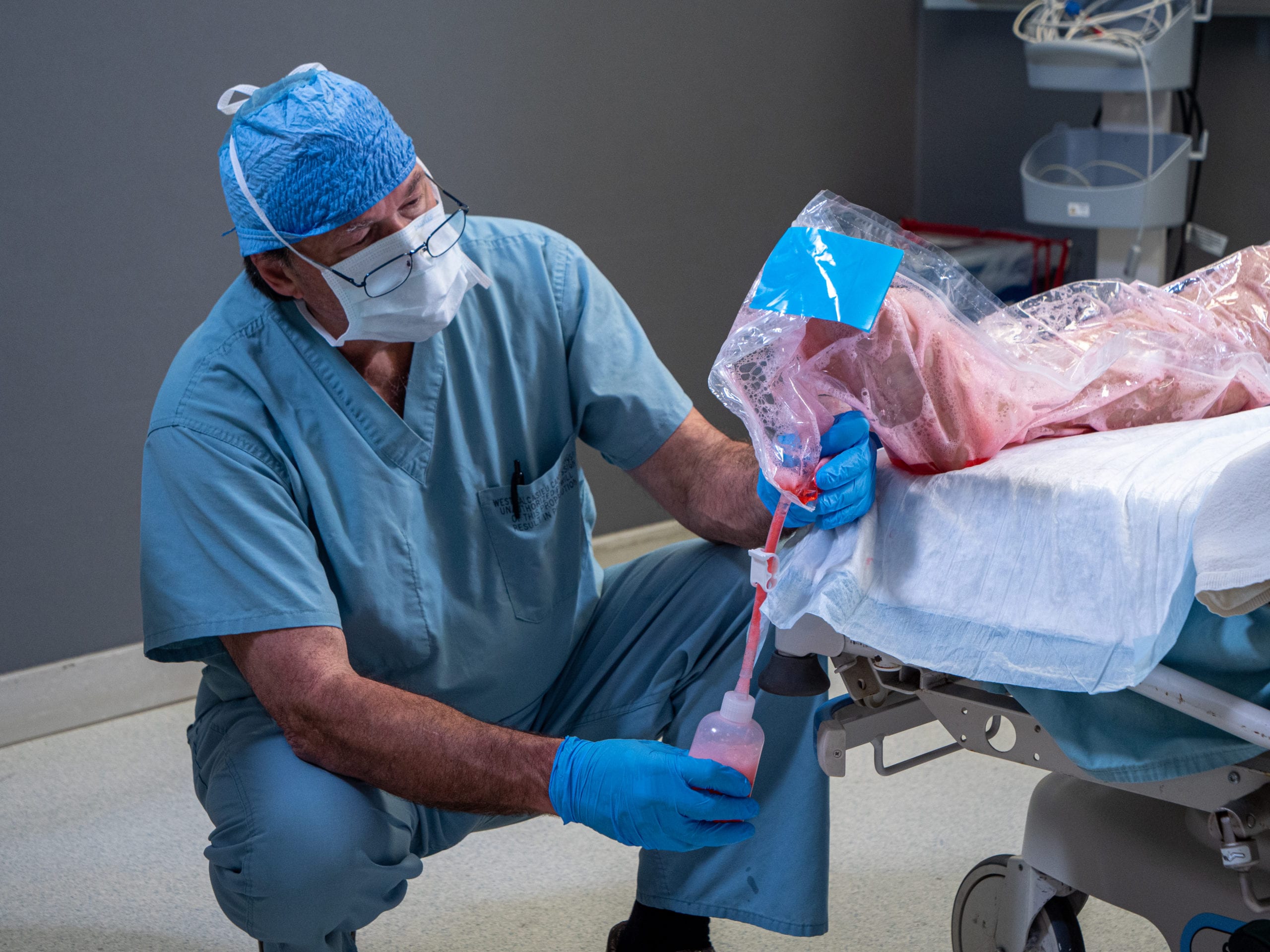

Partnering with the Army
In April 2020, PrepTech was made aware by LSU that the U.S. Army was also developing a Covid-19 Airway Management Isolation Chamber (CAMIC) similar in many ways to ISOCUBE™. After a series of collaborative discussions with the Army’s Tech Transfer office, Prep Tech was granted a license agreement to produce a commercially available CAMIC device; and then entered into a Cooperative Research and Development Agreement to co-mingle our designs. The Army affirmed the usage of ISOCUBE™ through months of collaborative discussions and prototype development.
Into the Future
PrepTech’s team continues to innovate and partner with like-minded companies who desire to help the global healthcare community become safer and more efficient. Please contact us to share ideas and to learn more!
